Compliance at iCenna
At iCenna, we understand that compliance is not optional—it's essential. As a trusted provider of digital health solutions across Saudi Arabia and the Gulf region, we are committed to meeting and exceeding all regulatory, legal, and ethical standards to ensure the safety, privacy, and integrity of our clients' data and operations.

Our Commitment
We adhere to local and international regulations that govern healthcare IT systems, ensuring full alignment with national healthcare regulations and global best practices.
Saudi Health Council Guidelines
Complete adherence to national healthcare standards and protocols
SDAIA Regulations
Data & Artificial Intelligence Authority compliance for data governance
NCA Cybersecurity Framework
National Cybersecurity Authority standards for digital security
HIPAA Standards
Health Insurance Portability and Accountability Act (where applicable)
ISO 27001 & ISO 27799
Information Security & Health Informatics international standards
Key Areas of Compliance
Comprehensive compliance coverage across all critical aspects of healthcare technology
Data Privacy & Security
We prioritize patient confidentiality by implementing end-to-end encryption, role-based access, and data residency within Saudi Arabia as mandated by SDAIA and the NCA.
End-to-end encryption for all patient data and communications
Role-based access control with multi-factor authentication
Data residency within Saudi Arabia as mandated by SDAIA and NCA

Clinical Safety & Accuracy
All our systems are designed to support clinical decision-making while minimizing medical errors, in line with MoH and CBAHI requirements.
Clinical decision support systems with evidence-based recommendations
Medical error prevention through automated alerts and validations
Full compliance with MoH and CBAHI clinical safety requirements
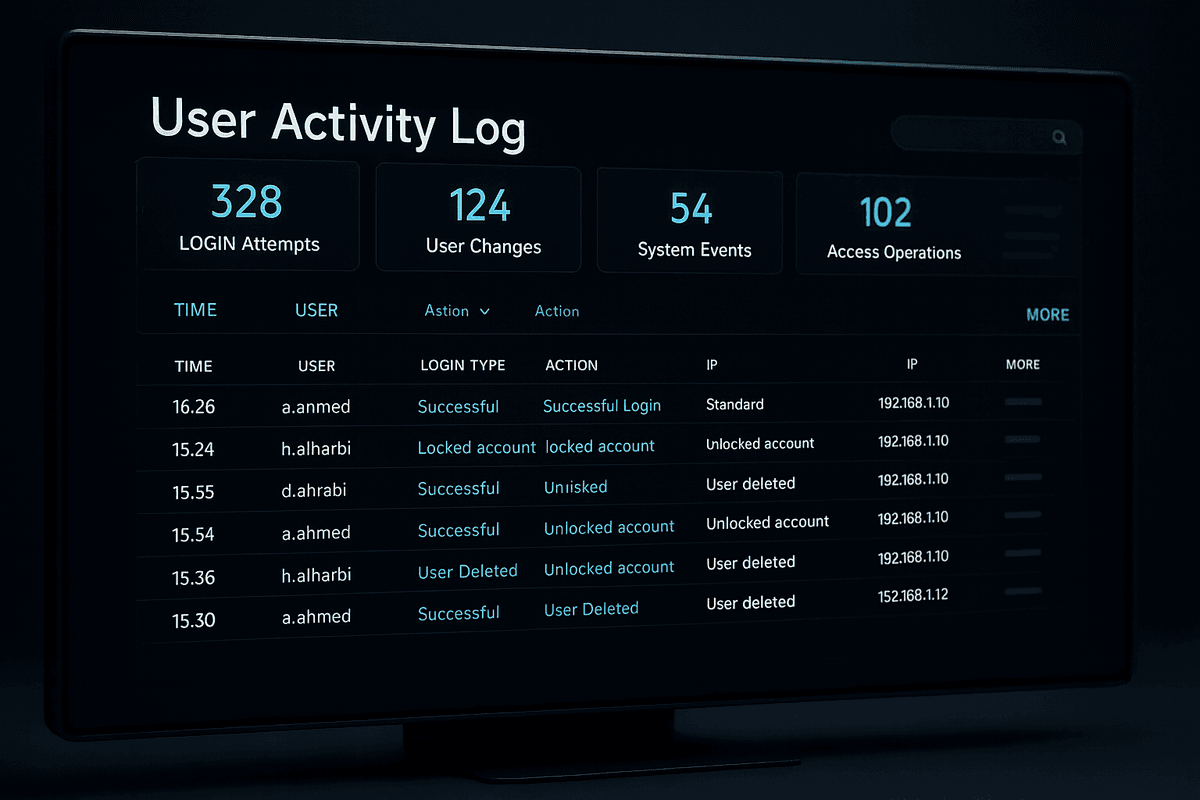
Audit & Traceability
We provide comprehensive logging, audit trails, and user activity reports to support regulatory audits and internal governance.
Comprehensive logging of all system activities and user actions
Detailed audit trails for regulatory compliance and internal reviews
Real-time user activity monitoring and reporting capabilities
Certifications & Frameworks
iCenna products and platforms are developed following international standards and best practices
ISO/IEC 27001
Information Security Management
OWASP Top 10
Secure Application Development
SDAIA Compliance
AI Ethics & Data Governance
Continuous Compliance Monitoring
We don't just implement compliance — we maintain it actively. Through regular internal audits, penetration testing, and third-party reviews, we continuously evaluate and upgrade our systems to adapt to evolving regulations and cybersecurity threats.
Internal Audits
Regular comprehensive reviews of all systems and processes
Penetration Testing
Ongoing security assessments to identify vulnerabilities
Third-Party Reviews
Independent validation of our compliance measures
Continuous Upgrades
Proactive adaptation to evolving regulations and threats


